SDV sucks
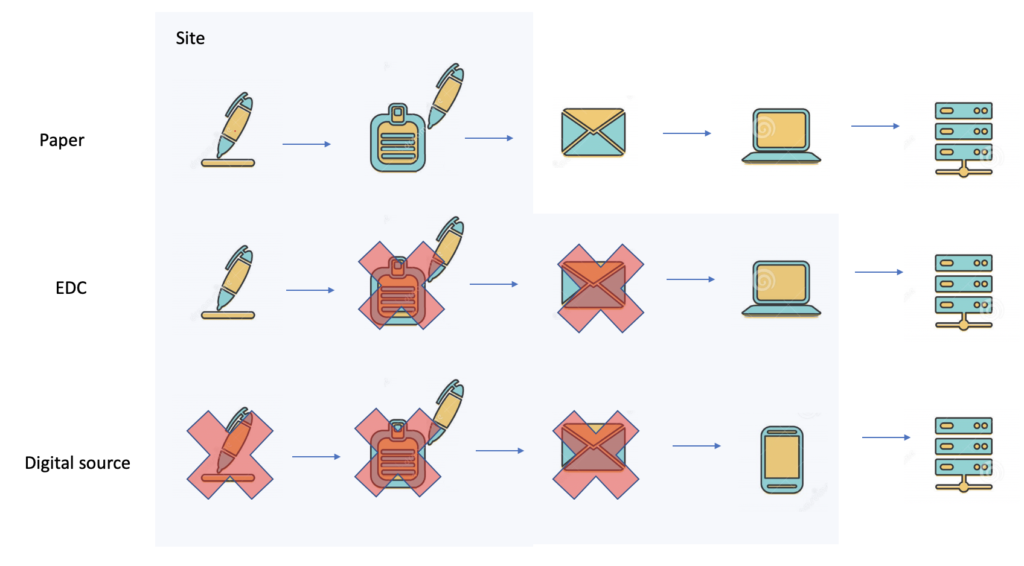
How professional services delay cures for patients. Why SDV Sucks In the early 50s of the previous century, nobody knew that 70 years later clinical trials could be run using a phone. Clinical trials that were performed 70 years ago used paper forms. By the late 70s, batch-driven data processing systems were introduced. Paper forms […]
Urban medical legends
Because I was trained as a solid-state physicist I am skeptical of many medical claims – including the efficacy of digital health apps. Gina Kolata wrote this post last week. I’ll let you decide for yourself. You might assume that standard medical advice was supported by mounds of scientific research. But researchers recently discovered that […]
What takes precedence? GCP or hospital network security?
This is a piece I wrote a while back on my medical device security blog – Cybersecurity for medical devices. One of the biggest challenge of using connected medical devices in clinical trials is near real-world usage of devices that are not commercially-ready. We have a couple of customers that are performing clinical trials of […]
4 strategies to get connected medical devices faster to FDA submission
Introduction Better designs, site-less trials, all-digital data collection and PCM (patient compliance monitoring) can all save time and money in connected medical device clinical trials. This article will help you choose which strategies will be a good fit to help you validate your connected medical device and its intended use for submission to FDA. What […]
Israel Biomed 2019-the high-social, low stress STEM conference
Impressions from Biomed 2019 in Tel Aviv This week was the annual 3 day Biomed/MIXiii (I have no idea what MIXiii means btw) conference in Tel Aviv. The organizers also billed it as the “18th National Life Science and Technology Week” (which I also do not know what that means). This was a particular difficult […]
Perverse incentives
The perverse incentive for the high costs of medical devices and delay to market The CRO outsourcing model and high US hospital prices result in higher total CRO profits via higher costs to companies developing innovative medical devices. These costs are passed down to consumers after FDA clearance. We’ll take a look at the cost […]
The 3 tenets for designing a clinical data management system
Abstract: This post reviews the importance of 1) proper study design, 2) good data modeling and 3) realistic estimation of project timetables. The article concludes with a discussion of eSource and attempts to dispel some of the myths including how DIY EDC study build save time (they don’t). Enjoy! The trend of DIY: good for […]
Important EDC features for medical device clinical trials
Medidata Rave and its CTMS companion product iMedidata are a far more comprehensive solution than OpenClinica but when you choose EDC software for medical device clinical trials, you enter a realm of unique requirements involving connectivity, security, privacy, API integration and specific interfaces to hardware. Electronic data capture software (EDC software) systems have demonstrated that […]
Why medical device studies need business controls
There are some interesting analogies between cyber security and medical device clinical trials from a risk management perspective. Both areas are complex, vulnerable to human exploits and may result in loss of data. Medical device trials are not exempt from unexpected human behavior. Despite this concern, I find it significant that guidance for remote-risk-based monitoring […]
The key is not first to eSource, the key is smart to market
This post is not for the Pfizers, Novartis, Merck and GSK giants of the life science industry. Its for the innovators, the smaller, creative life science companies that are challenged by the costs, the regulatory load and complexity of executing a clinical trial. This post is dedicated to the startup entrepreneurs of the world. Building an EDC […]
