What is easier to detect in your study – Slow-moving or fast moving deviations?
This post considers human frailty and strengths.
We recently performed a retrospective study of the efficacy of Flaskdata.io automated study monitoring in orthopedic trials. An important consideration was the ability to monitor patients who had received an implant and were on a long term follow-up program. Conceptually, monitoring small numbers of slow-moving, high-risk events is almost impossible to do manually since we miss a lot of what goes on around us, and we have no idea that we are missing so much. See the invisible gorilla experiment for an example.
One of patients in the study had received a spinal implant and was on a 6 month follow-up program dived into a pool to swim a few laps and died by drowning despite being a strong swimmer. Apparently, the pain caused by movement of the insert resulted in loss of control and a severe adverse event. The patient had disregarded instructions regarding strenuous physical activity and the results were disastrous.
It seems to me that better communications with the patients in the medical device study could have improved their level of awareness of safety and risk and perhaps avoided an unnecessary and tragic event.
Subjects and study monitors are both people.
This might be a trivial observation but I am going to say it anyhow, because there are lessons to be learned by framing patients and monitors as people instead of investigation subjects and process managers.
People are the specialists in their personal experience, the clinical operations team are the specialists in the clinical trial protocol. Let’s not forget that subjects and study monitors are both people.
Relating to patients in a blinded study as subjects without feelings or experience is problematic. We can relate to patients in a personal way without breaking the double blinding and improve their therapeutic experience and their safety.
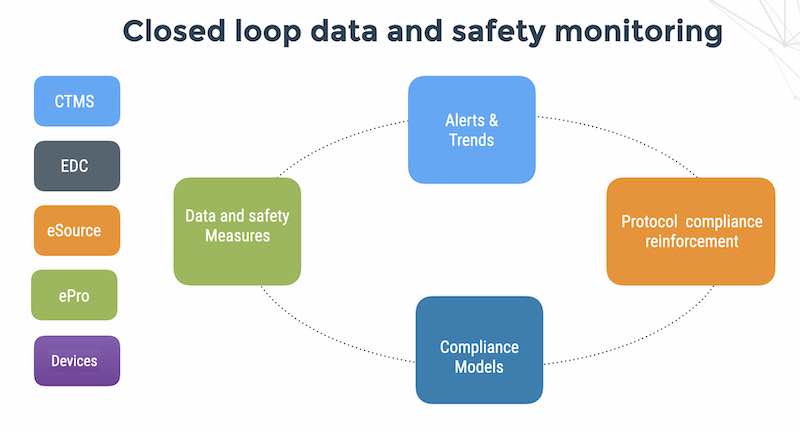
We should relate to study monitors in a personal way as well, by providing them with great tools for remote monitoring and enable them to prioritize their time on important areas such as dosing violations and sites that need more training. We can use analytics of online data from the EDC, ePRO and eSource and connected medical devices in order to enhance and better utilize clinical operations teams’ expertise in process and procedure.
A ‘patient-centered’ approach to medical device clinical trials
In conditions such as Parkinsons Disease, support group meetings and online sharing are used to stay on top of medication, side effects, falls and general feeling of the patient even though the decisions on the treatment plan need to be made by an expert neurologist / principal investigator and oversight of protocol violations and adverse events is performed by the clinical operations team. There are many medical conditions where patients can benefit by taking a more involved role in the study. One common example is carpal tunnel syndrome.
According to the findings of an August 3rd, 2011 issue of the Journal of Bone and Joint Surgery (JBJS), patients receiving treatment for carpal tunnel syndrome (CTS) prefer to play a more collaborative role when it comes to making decisions about their medical or surgical care.
Treatment of carpal-tunnel syndrome which is very common and also extremely dependent upon patient behavior and compliance is a great example of the effectiveness of “shared decision-making, or collaborative, model” in medicine, in which the physician and patient make the decision together and exchange medical and other information related to the patient’s health.
As the article in JBJS concludes:
“This study shows the majority of patients wanted to share decision-making with their physicians, and patients should feel comfortable asking questions and expressing their preferences regarding care. Patient-centered care emphasizes the incorporation of individual styles of decision making to provide a more patient-centered consultation,” Dr. Gong added.
In a ‘patient-centered’ approach to medical device clinical trials, patients’ cultural traditions, personal preferences and values, family situations, social circumstances and lifestyles are considered in the decision-making process.
Automated patient compliance monitoring with tools such as Flaskdata.io are a great way to create a feedback loop of medical device clinical data collection, risk signatures improvement, detection of critical signals and communications of information to patients. Conversely, automated real-time patient compliance monitoring is a a great way of enhancing clinical operations team expertise.
Patients and study monitors are both people.