CLINICAL TRIAL READINESS ASSESSMENT

Are you really ready to run a clinical trial? How can you best assess your clinical trial readiness? We work with C-level executives and management boards and understand their objectives, constraints and timelines. Do this before buying services for clinical data management, EDC, ePRO and biostatistics. Using a fixed price, fixed delivery model, our clinical trial readiness […]
Assuring protocol compliance without checklists

At a site monitoring visit, the CRA works on assuring protocol compliance. Assuring protocol compliance is one of the 3 pillars of GCP: The 3 pillars of GCP (good clinical practice) 1. Patient safety 2. Protocol compliance 3. Data quality (We note that setting the focus on the primary clinical and safety end-points results in […]
Home alone and in a clinical trial

1 in 7 American adults live alone COVID and social isolation is part of our lives. During COVID, it became easier to recruit patients for clinical trials. Like increased online shopping during social isolation of COVID? How do deal with patients who are home alone and in a clinical trial? But — somehow, that seems […]
Preventing drug counterfeiting

Counterfeiting is old as money itself. Drug counterfeiting is no exception. In this post, we’ll share an analytical approach that you can use to estimate the risk of drug counterfeiting to your drug products. Why are Medicines a Target for counterfeiting? Pharma is a highly regulated sector. Why do drugs attract counterfeiters? Fakes can be […]
What risks really count for your clinical trials?

Is there a “black-box” risk management solution for your clinical trial? What clinical trial risks are the most important for you and your company? Risk mitigation and risk management for your clinical trials What clinical trial risks really count for your company? No question is more important for implementing an effective program of risk-mitigation and […]
Dealing with information junkies in decentralized clinical trials

DecentraIized clinical trials software vendor Medable is doing an outstanding job in understanding and executing an online work-flow between sites and patients at home.. This understanding and execution is a necessary but insufficient condition for the success of decentraIized clinical trials. At their core, decentraIized clinical trials and hybrid studies have the same issues as any clinical research project. These […]
Preventing patient data leaks

6 ways to protect patient data in your eClinical and digital health applications Patient data leaks is much more than patient privacy. Patient data leaks require a more complete approach to threat mitigation of patient data leakage, availability and data integrity attacks. Since 2019, we see rapidly increased use of decentralized clinical trials, hybrid trials, […]
How to ensure patient adherence in decentralized clinical trials

Patient adherence in clinical trials without technology How can you assure patient adherence to the protocol, when you don’t see the patient? Life science companies are increasingly turning to decentralized clinical trial models. This means that patients are home alone. They use ePRO to record side effects and outcomes from the treatment. With 10X more […]
How to meet the 10 top challenges in Phase 1 clinical trials

Phase 1 challenges are unlike larger Phase 2, Phase 3 studies. The science is still unsure. The the clinical operations team at a startup may still be under construction. In this post, I’ll share our experiences at flaskdata.io with early stage drug and device vendors doing their first Phase 1 safety study. You’ll see a […]
SDV sucks
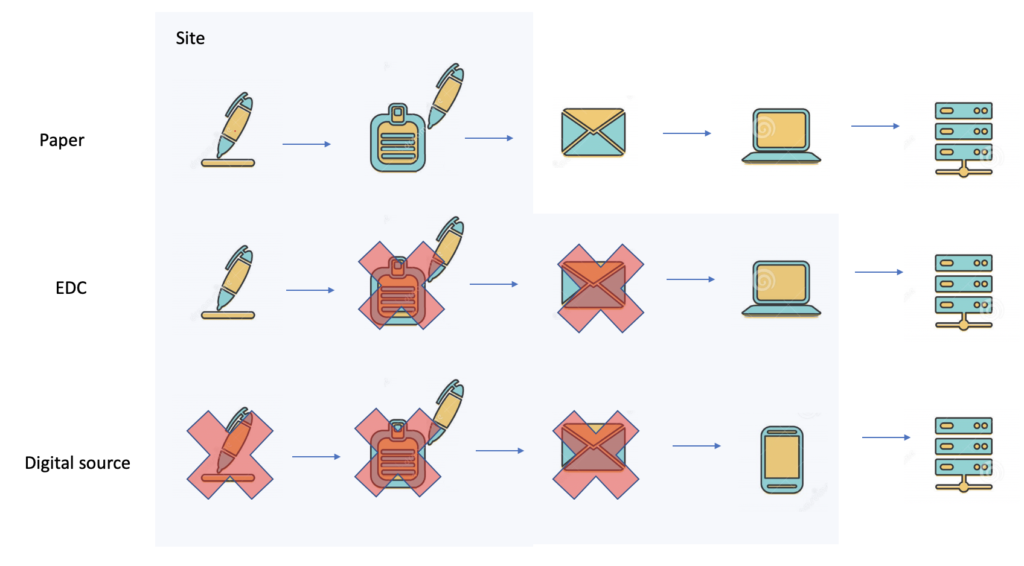
How professional services delay cures for patients. Why SDV Sucks In the early 50s of the previous century, nobody knew that 70 years later clinical trials could be run using a phone. Clinical trials that were performed 70 years ago used paper forms. By the late 70s, batch-driven data processing systems were introduced. Paper forms […]
